Abstract
Introduction
The practice of indefinite tyrosine kinase inhibitor (TKI) provision for Chronic Myeloid Leukaemia (CML) has remained unchallenged and the ability of TKIs to eradicate the CML clone is still largely unknown. Hence, a multicentre observational study involving major hospitals in Malaysia to observe the clinical practice to End TKI In CML (EnTIC) was performed. This study aims to determine the molecular response to TKI cessation in CML patients by close monitoring of BCR-ABL1.
Materials and Methods
A total of 88 adult patients diagnosed with CML in chronic phase and receiving first line TKI (imatinib, nilotinib or dasatinib) for at least four years with sustained MR4 (IS: 0.01%) for at least 2 years were recruited from January 2021 till July 2022. Close clinical review, and monthly BCR-ABL1 quantification analysis using real-time polymerase chain reaction for molecular monitoring were carried out.
Results
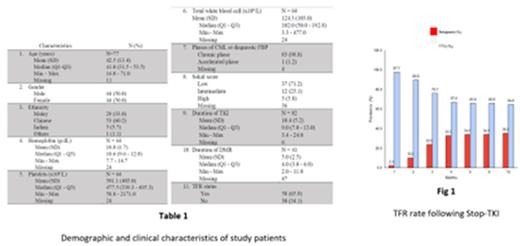
Eighty-eight patients with equal gender distribution (44 male and 44 female) and a median age of 41 years old (range, 31.5-53.5 years old) were included in the analysis. Chinese (60.2%, n =53) were the most prevalent ethnic group, followed by Malay (33%, n =29), and Indian (5.7%, n=5). 82.8% (n=64) of the patients had high presenting white blood cell count (>30x10⁹/L), with a median presenting white blood cell count of 102x109/L. Of the 64 patients, 38 of them (59.3%) had thrombocytosis (>450x10⁹/L). Majority of these patients (71.2%, n=37) presented with low Sokal score. First-line TKIs were administered to all patients throughout the course of treatment, with a median duration of 9 years (range: 7-13 years) and a median of 4 years for sustained deep molecular response (DMR) (range 3-6 years) was achieved. With a median follow-up of 14 months (range, 1-18 months) after stopping TKI, 57 patients (64.8%) remain in treatment-free remission (TFR). Thirty-one patients failed TFR, with a median duration of 2 months (range 1-5 months). Patients who remained in TFR at 166 days (5.5 months) and beyond were likely to achieve long term TFR in the following months (Fig 1).
Conclusion
The preliminary findings of EnTIC study demonstrate that a clinically significant percentage of patients were able to remain in MMR after stopping TKI. We observed the likelihood of breakthrough MMR during TFR trial is most often within the first 6 months of stopping TKI. Nonetheless, a longer follow-up duration and analyses of TFR data in EnTIC will be important to further evaluate the patients, and identifying clinically significant characteristics to predict disease relapse before stopping treatment.
Disclosures
Selvaratnam:Apellis Pharmaceuticals: Consultancy. Tan:Novartis: Honoraria, Research Funding; BMS: Honoraria; Amgen: Honoraria; MSD: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal